Color Trading Sp. z o. o. Asia-Pacific site
- English
Please select your Region.
Please select your Region.
Jun 11, 2013
ARKRAY, Inc. (herein, ARKRAY) will sell the BNP test device RapidPIA together with the dedicated reagent Rapid Chip BNP. BNP1) is a marker useful in the clinical identification of heart failure. The system is a compact and lightweight method to rapidly and easily detect this disorder.
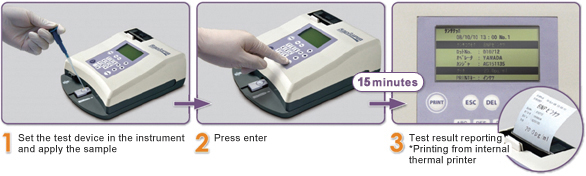
Rates of heart failure have increased in recent years. In line with this, a marker for heart failure, BNP, is being tested at increasing frequencies. Tests using RapidPIA and Rapid Chip BNP take just 15 minutes from blood sampling and forms a simple and quantitative way to test for BNP. Results can be immediately output for patients suspected of having heart failure in the ambulance or in an outpatient setting which helps improve treatment decisions and care.
ARKRAY has been developing POCT (Point of Care Testing)2) products for many years for distribution to clinics and smaller hospitals. This business partnership will strengthen ARKRAY's product portfolio and serve to reinforce diagnosis and treatment support for lifestyle-associated disease in the clinical field.
1) BNP (Brain Natriuretic Peptide)
This is a hormone mainly secreted into the blood from the heart ventricles. Secretion increases as stress is placed on the heart resulting in a rise in BNP in the blood. This is useful as a marker of heart failure as it reflects the level of stress the heart is under.
2) POCT (Point of Care Testing)
This refers to testing performed at GP or specialist clinics/ wards and other outpatient centers near to the patient (the bedside).
•Simple operation
Rapid and quantitative measurement of BNP from whole blood or plasma without pre-treatment
•Rapid measurement
Measurement in just 15 minutes. A built-in thermal printer provides rapid reporting of results.
•Compact size and light weight
Compact (A4 footprint) and light (1.4kg) allowing installation in clinical and areas with little space.
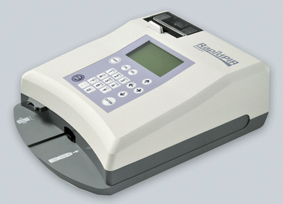
BNP Measurement Device RapidPIA®
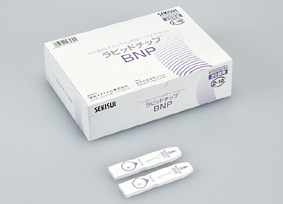
BNP rapid measurement diagnostic reagent
Rapid Chip® BNP
Quick and simple 3 step procedure for output of measurement results.
| Commercial name | RapidPIA® |
|---|---|
| Release date | Monday, 17 June 2013 |
| Specifications | |
| Measurement time | 15 minutes |
| Detection method | Reflected light intensity method |
| Display | LCD 8 lines |
| Printer | Thermal printer |
| Interface | USB, RS-232C |
| Power supply voltage | AC100V (50/60Hz) 0.4A |
| External dimensions | 205 x 275 x 96mm |
| Weight | Approx. 1.4kg |
| Operation temp./ humidity | 15-30C, 70% or less (non-condensing) |
| Sale price | JPY500,000 (requested delivery price) |
| Medical Device Applic. No. | 22B1X00008D00015 |
| Class | General medical device; Special Maintenance Control Medical Device |
| Commercial name | Rapid Chip® BNP |
|---|---|
| Release date | Monday, 17 June 2013 |
| Packaging | Test device x 20 (accessory: CAL card x 1) |
| Storage method & shelf life | 2-10C, 1 year after manufacture |
| Compatible sample | Plasma or whole blood with EDTA-2Na or EDTA-2K (120µL) |
| Certification number | 221ADAMX00004000 |
| Drug efficacy classification | In vitro diagnostic reagent |
This product will be sold through ARKRAY Marketing, Inc. (ARKRAY, Inc.'s distributor in Japan). This product is only available in Japan and is not sold in other countries.
![]()